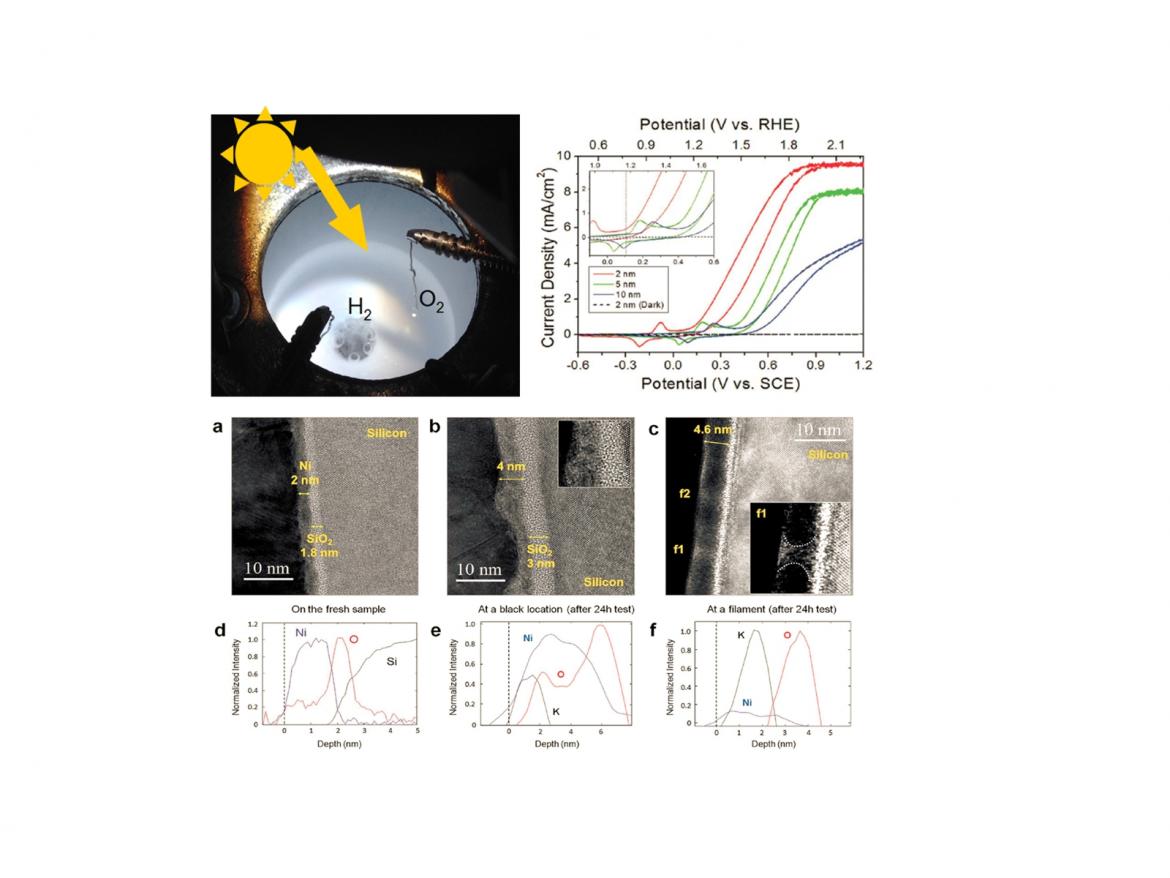
Splitting water into hydrogen and oxygen is a key component of clean fuel and chemical synthesis from solar energy. The principle is that solar-light-driven electron-hole pairs in a semiconductor make redox reaction at the interface between the semiconductor surface and aqueous solution, resulting in water-splitting to H2 and O2.
Thus, a semiconductor used as the light-excitation site is important to decide the efficiency of solar-to-hydrogen conversion. Many studies on oxide semiconductor such as TiO2, WO3, BiVO4, and ZnO have been reported. However, they have drawbacks such as large band-gap or unstability.
These hinder light absorption in the visible light range, producing only modest amounts of hydrogen and oxygen. Moreover, often the chemical reactions decay after a few minutes due to surface corrosion. To overcome these limits, the most advanced water-splitting cells use a catalytic and protective coating on the semiconductor surface. Several metal-based catalysts, including Ru, Ni, NiOX, Ir, Cu, CuOX, MnOX and Co have been demonstrated to enhance the activity of semiconductor photoelectrodes, achieving current densities of up to tens of mA cm-2. However, in order to avoid reduction of the transmittance of light to the semiconductor, the thickness of the metal coating has to be very low, on the order of a few nm, and therefore the stability remains a major concern.
The activity at IMM is based on the use of silicon as low cost, high efficient light absorber semiconductor. We study both the photoanode or the photocathode, using different structures.
We have studied the ageing mechanisms that produce a decay in activity and device failures. The cells we have employed as photoanode are metal-oxide-semiconductor devices fabricated by depositing a thin Ni film on oxidised silicon. The resulting photoelectrodes were tested under potassium hydroxide (KOH, pH =14) in a three-electrode system, using a saturated calomel electrode (SCE) and a stainless steel counter electrode.
The cyclic voltammograms measured for different Ni thickness shows the largest saturated current and the lowest onset potential for the 2 nm thick layer. However, due to the reduced thickness, these electrodes become unstable after 10 hours, while the photoanodes with 5 or 10 nm of Ni remain stable up to more than 10 days.
The study of the degradation mechanisms has evidenced first, the oxidation of the Ni layer, than the formation of pinholes through which the KOH solution was able to reach the Si/SiO2 interface and then the etching of silicon.
The activity is now focused on the silicon based photocathode, using a heterojunction solar cell, in order to be able to produce high current with a long term stability.
Contact: Stefania Privitera
This activity is in collaboration with prof. Mario Lanza, Institute of Functional Nano & Soft Materials, Soochow University, China.
Recent publications:
- Ageing mechanisms of highly active and stable nickel-coated silicon photoanodes for water splitting, T. Han, Y. Shi, X. Song, A. Mio, L. Valenti, F. Hui, S. Privitera, S. Lombardo, M. Lanza, Journal of Materials Chemistry A, vol. 4, 8053 (2016)
- 3C-SiC Polycrystalline Films on Si for Photovoltaic Applications, S. Privitera, V. Brancato, D. Spadaro, R. Anzalone, A. Alberti, F. La Via, Materials Science Forum 821, 189-192 (2015)


