The realization of electrochemical cells for the synthesis of green fuels, such as hydrogen, by water splitting, or ammonia by reaction of nitrogen gas with water or green hydrogen, represents an important step towards clean energy innovation, since it enables distributed green fuels production, by using renewable energy sources. The development of fuel cell systems, in which the fuel is employed to get back electricity, allows to complete the cycle with zero carbon emission, mitigating the intrinsic fluctuation of renewables.
To this aim, within the H2020 TELEGRAM project*, electrochemical systems for ammonia production and oxidation will be developed.
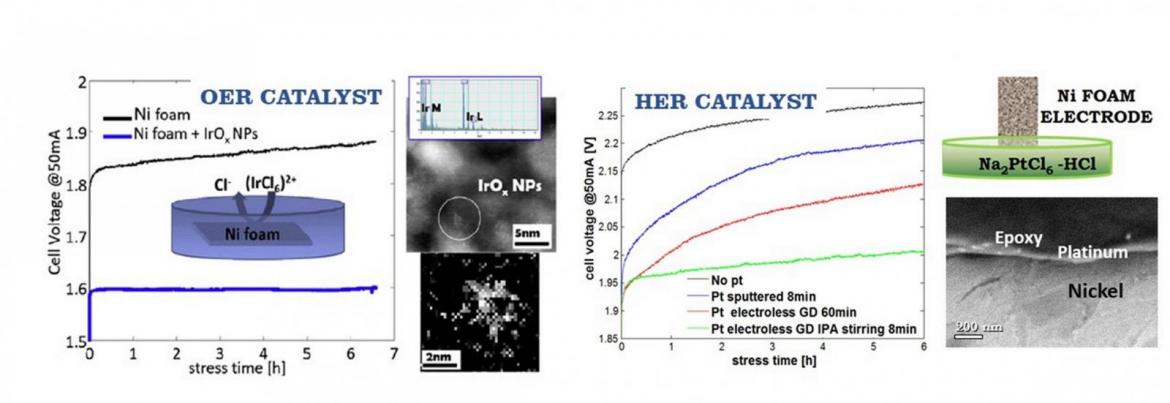
Electrochemical cells require the integration of catalysts into complex multifunctional systems including gas diffusion electrodes, electrolyte, membrane and current collectors. Moreover, different aspects have to be taken into account, such as the liquid and gas fluxes into the cell, as well as the mechanical strength and corrosion resistance of the constituent parts.
Among catalysts, nanostructured materials prepared with different processes, from sputtering to electroless or electrochemical deposition, as well as chemical co-precipitation, are employed for this activity, because of their large surface-to-volume ratio. Various catalysts are integrated into gas diffusion electrodes, that can be 3D structured metals, such as Ni or Cu foam, or carbon based electrodes (graphene, carbon clothes, carbon paper). Both alkaline and polymer electrolyte are studied, in order to evaluate the effect of the entire system on the final efficiency.
Contact: Stefania Maria Serena Privitera
*TELEGRAM project has received funding from the European Union's Horizon 2020 Research and Innovation programme under grant agreement No 101006941.


