Lead-halide perovskites are outstanding materials for photovoltaics, providing efficiencies of > 25 % in solar cells devices.1 The presence of lead, however, is raising serious concerns about potential treats to living organisms.2 The full replacement of Pb by Sn in MASnI3 (MA = Methylammonium) has been demonstrated to be an effective strategy,3 however, the performance of the resulting devices is limited by uncontrollable self p-doping and rapid degradation of the lattice into optically inactive tin(IV) phases.4
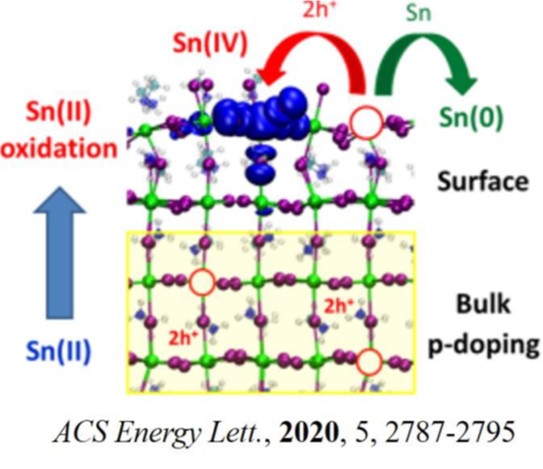
Due to the raising interest in these materials, we performed an extensive ab-initio defect chemistry analysis to understand the atomistic origins behind the poor performances of tin iodide perovskite solar cells. In doing so, we identify significant differences with MAPbI3 ascribed to the larger oxidation potential of MASnI3 and we show that tin vacancies can be easily formed in the latter, being the main responsible of the severe p-doping.5 We then understand, by means of simple models, that the oxidation of tin(II) into tin(IV) occurs mainly on the surface of the material, promoted by the presence of low coordinated Sn units.5-6
Thus, stabilizing tin-iodide perovskites may seem a desperate case because of the two (bulk and surface) oxidation channels related to the high band edge energetics. We finally illustrate possible strategies aimed at mitigating MASnI3 instability through tailored substitution of tin and iodine, with isovalent and aliovalent metals (B-site doping) and with different halides.
(1) Stranks, S. D.; Snaith, H. J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat Nano 2015, 10, 391-402.
(2) Li, J.; Cao, H.-L.; Jiao, W.-B.; Wang, Q.; Wei, M.; Cantone, I.; Lü, J.; Abate, A. Biological Impact of Lead from Halide Perovskites Reveals the Risk of Introducing a Safe Threshold. Nat. Comm. 2020, 11, 310.
(3) Noel, N. K.; Stranks, S. D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G. E.; Pathak, S. K.; Johnston, M. B., et al. Lead-free organic-inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061-3068.
(4) Dalpian, G. M.; Liu, Q.; Stoumpos, C. C.; Douvalis, A. P.; Balasubramanian, M.; Kanatzidis, M. G.; Zunger, A. Changes in charge density vs changes in formal oxidation states: The case of Sn halide perovskites and their ordered vacancy analogues. Phys. Rev. Mat. 2017, 1, 025401.
(5) Meggiolaro, D.; Ricciarelli, D.; Alasmari, A. A.; Alasmary, F. A. S.; De Angelis, F. Tin versus Lead Redox Chemistry Modulates Charge Trapping and Self-Doping in Tin/Lead Iodide Perovskites. J. Phys. Chem. Lett. 2020, 11, 3546-3556.
(6) Ricciarelli, D.; Meggiolaro, D.; Ambrosio, F.; De Angelis, F. Instability of Tin Iodide Perovskites: Bulk p-Doping versus Surface Tin Oxidation. ACS Energy Lett. 2020, 5, 2787-279

